Two COVID-19 Patients Who Received Plasma Therapy In Gujarat Show Incredible Improvement
Gujarat has become the latest state to start plasma therapy for COVID-19 patients. Two coronavirus patients admitted to the SVP Hospital in Ahmedabad were given plasma therapy and the condition of both are stable. The Indian Council of Medical Research ICMR has granted permission to conduct clinical trials on COVID 19 patients across public hospitals.
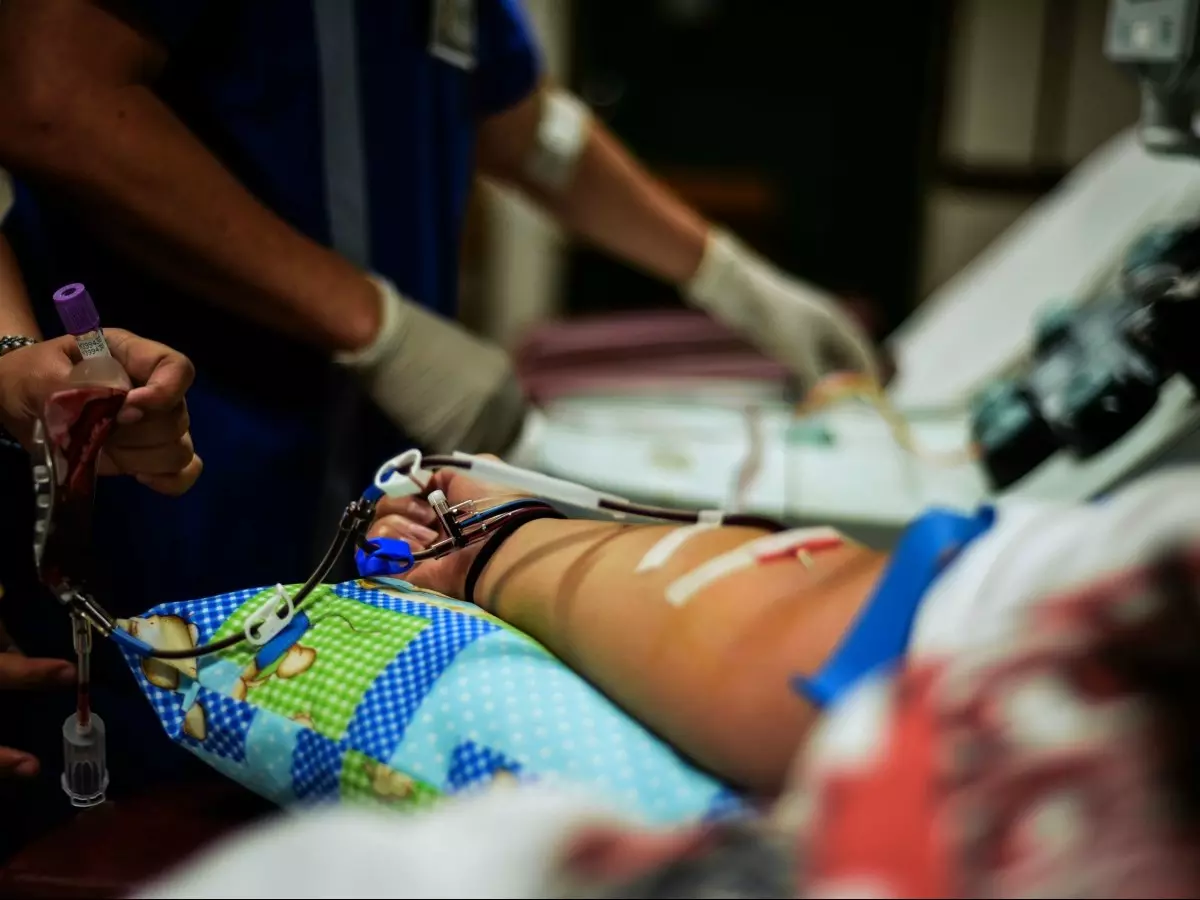
In what could be a game-changer in the fight against the COVID-19 outbreak, patients who have been given plasma therapy have shown signs of a significant improvement in their condition.
Gujarat has become the latest state to start plasma therapy for COVID-19 patients. Two coronavirus patients admitted to the SVP Hospital in Ahmedabad were given plasma therapy and the condition of both are stable, Principal Health Secretary Jayanti Ravi has said.
 AFP
AFP
Two doses of 200 ML convalescent plasma each from a recovered COVID-19 patient were administered to them.
The first donor was a 24-year-old who was discharged on April 6. The second donor was a 34-year-old woman who was discharged on March 29.
Earlier, a 49-year-old Delhi man who was administered plasma therapy in Saket Max Hospital has been taken off the ventilator support after his condition improved. He has now recovered and tested negative for coronavirus twice.
The Indian Council of Medical Research (ICMR) has granted permission to conduct clinical trials on COVID-19 patients across public hospitals to test the safety and efficacy of convalescent plasma therapy.
 AFP
AFP
Dr Asha Kishore, Director, Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) Thiruvananthapuram said that the ICMR has issued a new call for a multi-centre clinical trial across India and published an ICMR protocol, which is approved by DCGI.
"This proposal is for studying the effect of convalescent plasma therapy in moderate cases of COVID- 19. As the state doesn't have severe cases now, this new protocol for moderate cases is more feasible in Kerala, "she told ANI.
Asha Kishore said that COVID hospitals with blood banks satisfying the eligibility criteria for the trial can apply to ICMR.
 REUTERS
REUTERS
"If approved, they can register for the clinical trial. The hospitals under state government that treat COVID-19 cases have applied. Once the state government gets the approval, the transfusion medicine department of SCTIMST which had originally submitted its proposal to the state government may be able to collaborate with the state hospitals with the government's approval, " she said.
SCTIMST is an institute of national importance under the Department of Science and Technology. It had received the clearances from the ICMR to conduct clinical trials for plasma therapy. The plasma therapy uses antibodies from the blood of a recovered COVID-19 patient to treat another patient.
Till now in the country, it has been carried out to treat critically ill patients and now it will also be tested on moderate COVID-19 cases.
