Right Direction! Oxford University's Coronavirus Vaccine Moves To Next Stage Of Human Trial
University of Oxford has confirmed that they will be moving to the next level as they begin recruiting over 10000 people for the second phase of human trials. Last month the first phase of the trial had begun and over 1000 healthy adults aged 55 and under were recruited as volunteers. Now in the next phase more than 10200 people including over 70s and five to 12-year-olds will be enrolled in the study.
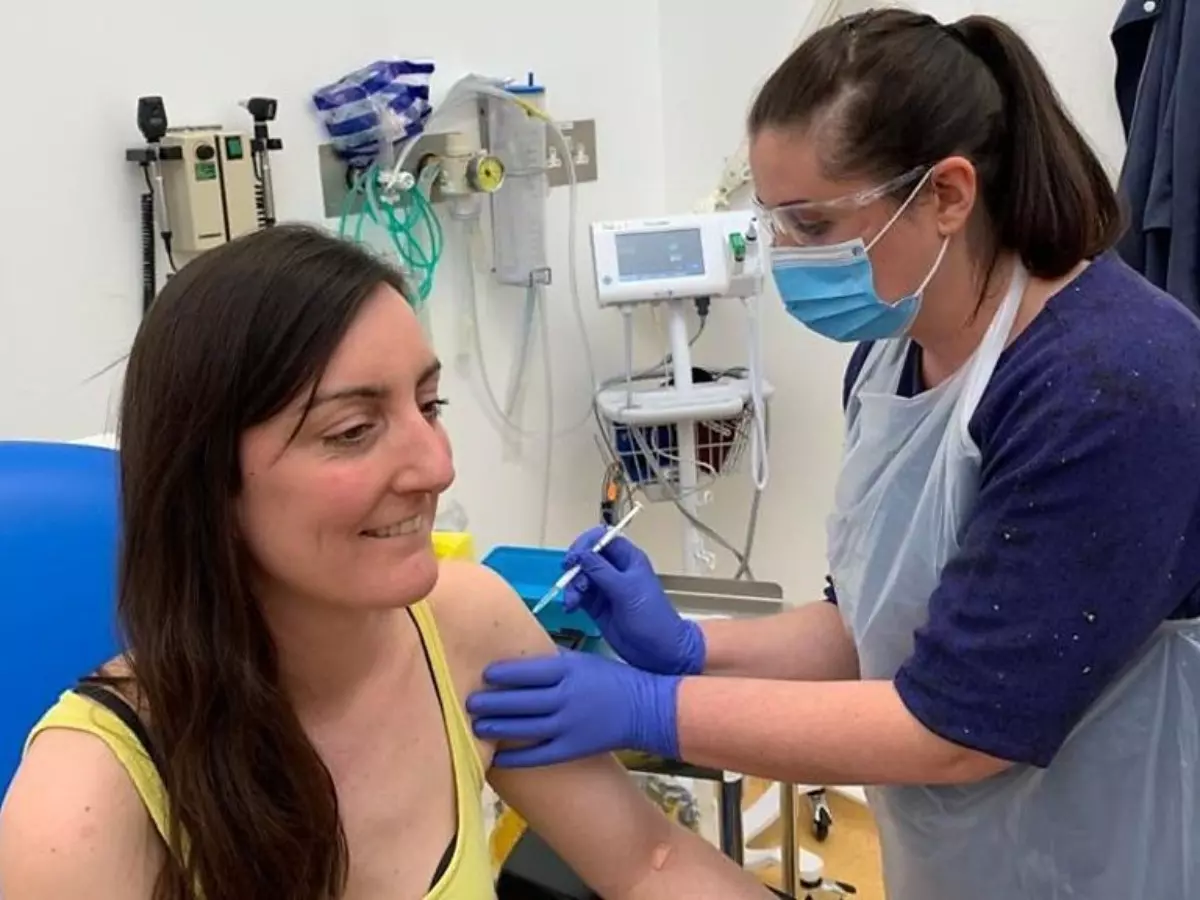
University of Oxford, which is in race to develop the coronavirus vaccine, has confirmed that they will be moving to the next level as they begin recruiting over 10,000 people for the second phase of human trials.
 BBC
BBC
Last month, the first phase of the trial had begun and over 1,000 healthy adults aged 55 and under were recruited as volunteers. Now in the next phase, more than 10,200 people, including over 70s and five to 12-year-olds, will be enrolled in the study, to see the effects on their immune system.
A recent study had found that the vaccine, named ChAdOx1 nCoV-19, had shown some promising results in a small study with monkeys.
The vaccine contains a weakened version of a virus that causes the common cold in chimpanzees. They then combined this virus called the adenovirus with a coronavirus protein called spike protein.
 Representational Image
Representational Image
For the latest set of volunteers, researchers will be assessing the immune response to the vaccine in people of different ages, to find out if there is variation in how well the immune system responds in older people or children.
The Phase III part of the study involves assessing how the vaccine works in a large number of people over the age of 18.
This group will assess how well the vaccine works to prevent people from becoming infected and unwell with COVID-19. Adult participants in both the Phase II and Phase III groups will be randomised to receive one or two doses of either the ChAdOx1 nCoV-19 vaccine or a licensed vaccine (MenACWY) that will be used as a ¡°control¡± for comparison, the university said.
 AP
AP
Mene Pangalos, Executive Vice President for BioPharmaceuticals R&D at AstraZeneca, which has a partnership with the university for the production of the vaccine if it is proved effective, was quoted by PTI as saying: ¡°The speed at which this new vaccine has advanced into late-stage clinical trials is testament to Oxford¡¯s ground-breaking scientific research.
¡°We will do everything in our power to engage with governments, multilateral organisations and partners around the world to increase production and distribution and ensure rapid, fair and equitable distribution of a globally accessible vaccine.¡±
The study aims to assess how well people across a broad range of ages could be protected from Covid-19 with this new vaccine. It will also provide valuable information on the safety aspects of the vaccine and its ability to generate good immune responses against the virus.
The team behind the vaccine have previously said they are aiming to have at least a million doses of a coronavirus vaccine by September this year. However, the UK government has repeatedly warned that there are no guarantees a vaccine will be discovered against the deadly virus.
The Oxford University trial is among several experimental vaccines being developed worldwide to try and combat the spread of Covid-19 and help lift strict restrictions on human movement in place in most countries.
