US To Start 12 To 15-Year-Old Kids Covid Vaccination This Week, What About India?
The Pfizer vaccine was declared safe by the Food and Drugs Administration after studies revealed that it safely offers strong protection to younger teens, based on a study that involved over 2,000 volunteers from the age group of 12 to 15.
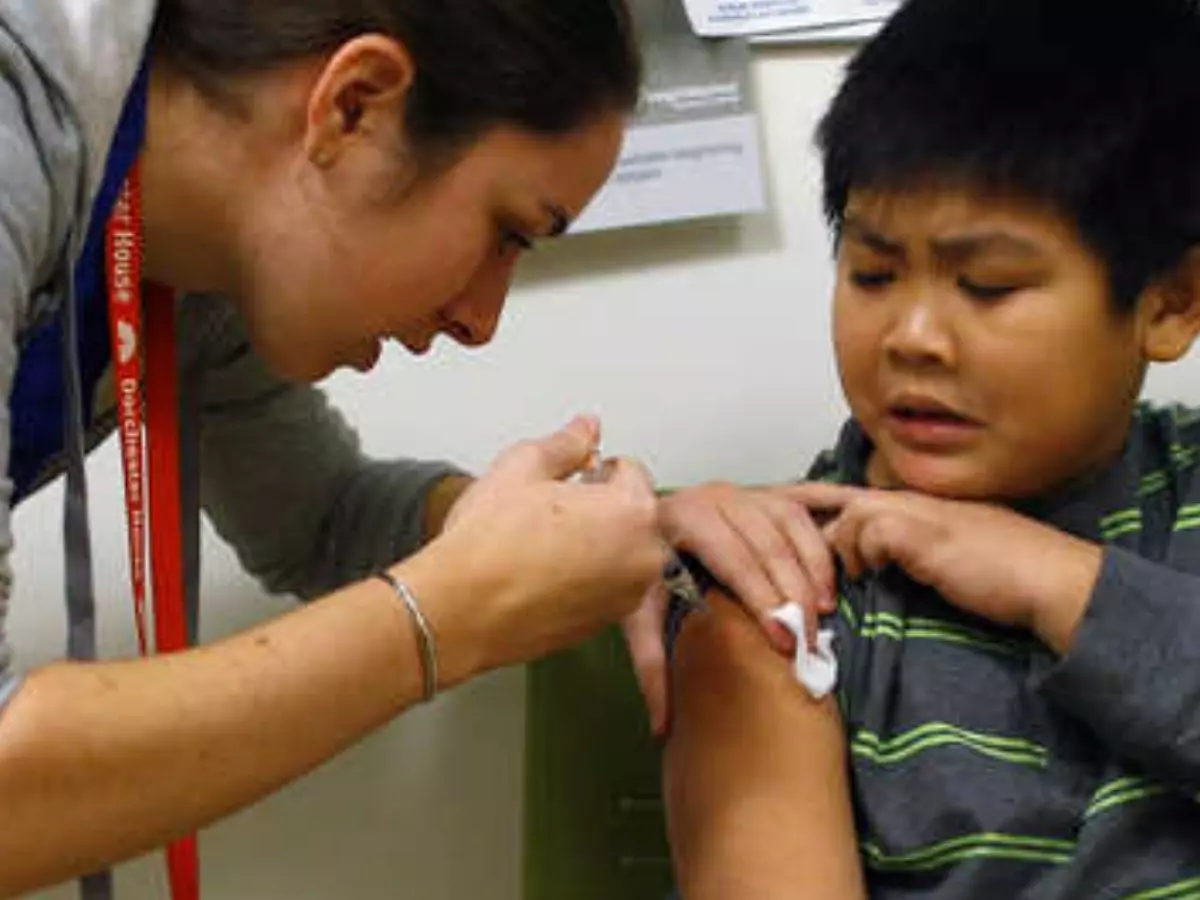
Kids between the ages of 12 to 15 could soon receive their COVID-19 jabs as US regulators on Monday approved the use of Pfizer¡¯s novel coronavirus vaccine for the aforementioned age group.
 Reuters
Reuters
Also Read: One Covishield Vaccine Dose Cuts Covid-19 Death Risk By 80%, Claims Study
Shots are expected to commence as soon as Thursday, after federal vaccine advisory issues recommendations for the use of the two-dose novel coronavirus vaccine in 12 to 15-year-olds, which is expected to arrive sometime on Wednesday.
President Biden said in a statement that it¡¯s a promising development in our fight against the virus, ¡°If you are a parent who wants to protect your child or a teenager who is interested in getting vaccinated, today¡¯s decision is a step closer to that goal."
Even though it¡¯s not yet completely greenlit in the US, kids of age 12 and above are being vaccinated with the Pfizer vaccine in Canada, with many nations vaccinating 16-year-olds and above with the vaccine.
The Pfizer vaccine was declared safe by the Food and Drugs Administration after studies revealed that it safely offers strong protection to younger teens, based on a study that involved over 2,000 volunteers from the age group of 12 to 15.
Also Read: India Has COVID-19 Vaccine On Its Mind, As 80 Lakh Register In 3 Hours
The study showed no COVID-19 infection among those who were fully vaccinated, compared to those given placebo. Moreover, researchers also found that kids developed higher levels of antibodies than earlier studies showed in young adults.
The kids were given a similar dose as the adults and they also experienced a similar set of side effects including flu-like fever, body aches, sore arms etc. However, it¡¯s not just Pfizer that¡¯s seeking approval for vaccinating teenagers. Moderna too with its preliminary study results has shown that 12 to 17-year-olds have shown strong protection with no serious side effects. Even Novavax has just commenced a study for 12 to 17-year-olds.
 Reuters
Reuters
What about children in India?
As of now, there isn¡¯t any vaccine approved in India to be administered to kids. The only approved vaccine that was undergoing trials with children was AstraZeneca¡¯s jab (known as Covishield in India), but the trials were paused a month ago after it was found to form blood clots in children. As of now, Pfizer-BuiNTech¡¯s jab is the only vaccine approved for use on 12 to 15-year-olds.
Also Read: Private Companies Can Import COVID-19 Vaccines In India: What This Means
While India doesn't have Pfizer vaccines yet, we could expect them to enter India soon as just last week, India¡¯s Central Drugs Standards Control Organisation has stated that any private entity can now import the COVID-19 vaccine if that has been approved by the drug regulatory authority and has received an import license for the vaccine.
Moreover, Pfizer is more than eager to send vaccine doses to India. Last week, Pfizer CEO, Albert Bourla even announced a medical aid worth $70 million to help with the second wave of the novel coronavirus that is wreaking havoc in our lives, while announcing that it is in talks with the government for an expedited approval pathway to make Pfizer-BioNTech vaccine available in India.
But even after we get the vaccine, we don¡¯t know how long it would take for approvals to allow it to be used on kids.
