Pfizer Is All Set To Begin Testing COVID-19 Vaccine In Children As Young As 12
Pfizer, one of four companies to have vaccines in advanced, Phase 3 clinical trials in the US, says it has enrolled close to 38,000 volunteers in its trial. More than 31,000 of them have received the second of two shots.
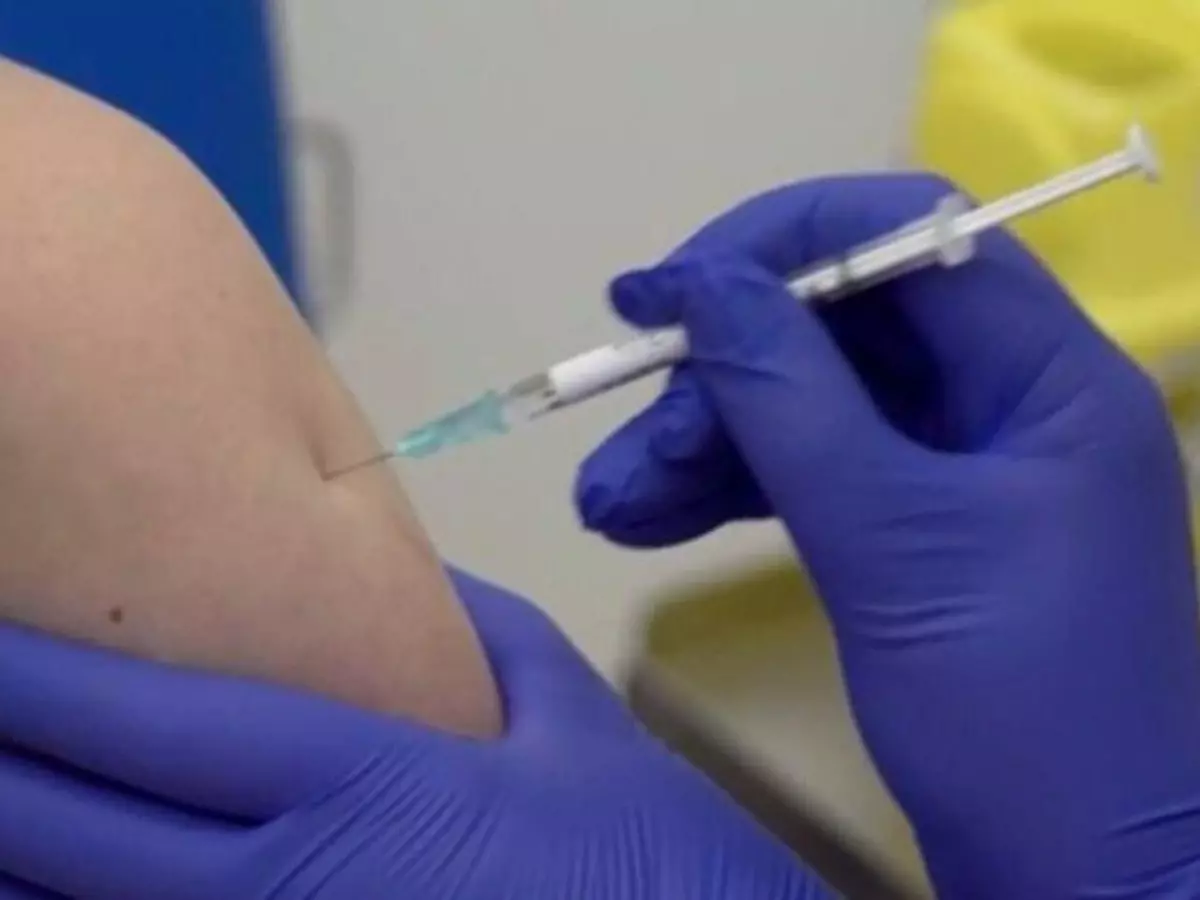
Drugmaker Pfizer has again modified the protocol for its late-stage study of its vaccine against coronavirus, this time to include more young participants.
The company said that it received permission from the Food and Drug Administration to include adolescents aged 12 through 15 in its global COVID-19 vaccine study.
 Reuters
Reuters
According to CNN, a team at Cincinnati Children's Hospital will begin vaccinating teenagers aged 16 and 17 this week, and will move to enroll 12-to 15-year-olds later, said Dr. Robert Frenck, director of the Vaccine Research Center at the hospital.
Also Read: COVID-19 Vaccine Trial Volunteers Experience Side Effects Like Headache, Fever & Exhaustion
The company confirmed on its website it has approval from the US Food and Drug Administration to enroll children as young as 12 in its trial
New York-based Pfizer originally planned for 30,000 participants, but in September expanded that to 44,000 people.
 Reuters
Reuters
That increase was made to boost diversity in the trial population, specifically by including 16- and 17-year-old teens, as well as stable patients with some common chronic infections: hepatitis B, hepatitis C and HIV.
Also Read: Clinical Trials Of A Second Russian Coronavirus Vaccine Have Proved Successful: Report
Pfizer's trial also includes significant numbers of Hispanic, Black, Asian and Native American participants, plus many people aged 56 through 85.
The diversity is aimed at providing information on how safe and effective the experimental vaccine is in people of different ages and backgrounds.
Pfizer, one of four companies to have vaccines in advanced, Phase 3 clinical trials in the US, says it has enrolled close to 38,000 volunteers in its trial. More than 31,000 of them have received the second of two shots.
 Reuters
Reuters
Late-stage vaccine trials initiated by Moderna, J&J and Novavax Inc are testing their respective candidates only in adults.
Also Read: Scared Of The Jab? Needle-Free Coronavirus Vaccine Trials Set To Start In Australia
AstraZeneca¡¯s U.K. vaccine trial, targeting more than 12,000 volunteers, will have one out of 11 subgroups with children 5 to 12 years of age. Chief Executive Officer Pascal Soriot said last month that tests on children had not yet started.
All Inputs PTI/Reuters
Disclaimer: While there have been several different types of treatments being given to COVID-19 patients across the world, there isn¡¯t any one drug that has worked as a sure-shot treatment yet. Don¡¯t self medicate/stock up and always consult your doctor/medical health professional.
